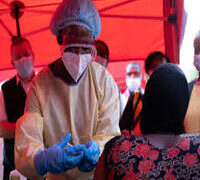
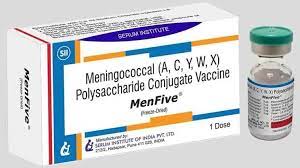
he Global Alliance for Vaccines and Immunizations says the MenFive vaccine is poised to revolutionize meningitis prevention efforts in Africa.
The Senior Programme Manager, Vaccine Implementation at Gavi (Meningitis programme lead), Ms Cassandra Quintanilla, said this in an interview with News Agency of Nigeria, on Monday in Abuja.
NAN reports that MenFive® is approved by the World Health organization for use in individuals between the ages of one to 85 years, and will initially be available for use in reactive vaccine campaigns for meningitis outbreaks.
Quintanilla said that unlike its predecessors, MenFive boasts of remarkable advantage of remaining stable outside of the cold chain for up to 12 weeks, making it a game-changer for regions with limited refrigeration resources.
According to her, this innovation promised greater flexibility in vaccine delivery, particularly in remote areas where maintaining a cold chain is challenging.
She said, “The MenFive vaccine, prequalified by the World Health Organization after extensive clinical trials, targets the five main serogroups of meningococcal meningitis prevalent in Africa, including serogroup X, previously unprotected by available vaccines.
“Its efficacy and safety have been demonstrated through comprehensive studies conducted across different countries, including Mali, India, the US, and the Gambia.”